The Impact of Sleep-Disordered Breathing on Body Mass Index (BMI): The Sleep Heart Health Study (SHHS)
 Thursday, December 8, 2011 at 11:41AM
Thursday, December 8, 2011 at 11:41AM Mark A. Brown, M.D. 1
James L. Goodwin, Ph.D.2
Graciela E. Silva, Ph.D, MPH.3
Ajay Behari, M.D.4
Anne B. Newman, M.D., M.P.H5,6
Naresh M. Punjabi, M.D., Ph.D.7
Helaine E. Resnick, Ph.D., M.P.H.8
John A. Robbins, M.D., M.S.H.9
Stuart F. Quan, M.D.2,10
1Department of Psychiatry, Kaiser Permanente, Portland, OR (markbrownmd@gmail.com);
2Sleep and Arizona Respiratory Centers, University of Arizona College of Medicine, Tucson, AZ(jamieg@arc.arizona.edu);
3College of Nursing & Health Innovation, Arizona State University, Tempe, AZ (Graciela.Silva@asu.edu);
4Pulmonary and Critical Care Associates of Baltimore, Baltimore, MD (ajaybehari@yahoo.com);
5Graduate School of Public Health, Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA
6Division of Geriatric Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA (NewmanA@edc.pitt.edu);
7Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD (npunjabi@jhmi.edu);
8American Association of Homes and Services for the Aging, Washington, DC (heresnick@gmail.com);
9Center for HealthCare Policy and Research, University of California, Davis, Sacramento, CA (jarobbins@ucdavis.edu);
10Division of Sleep Medicine, Harvard Medical School, Boston, MA (squan@arc.arizona.edu)
Address for correspondence and reprint requests: Stuart F. Quan, M.D., Division of Sleep Medicine, Harvard Medical School, 401 Park Dr., 2nd Floor East, Boston, MA 02215, Tel (617) 998-8842, Fax (617) 998-8823, Email: squan@arc.arizona.edu
Conflict of Interest Statement: None of the authors have conflicts of interest pertinent to the subject matter of this manuscript.
Reference as: Brown MA, Goodwin JL, Silva GE, Behari A, Newman AB, Punjabi NM, Resnick HE, Robbins JA, Quan SF. The impact of sleep-disordered breathing on body mass index (BMI): the sleep heart health study (SHHS). Southwest J Pulm Crit Care 2011;3:159-68. (Click here for PDF version of the manuscript)
Abstract
Introduction: It is well known that obesity is a risk factor for sleep-disordered breathing (SDB). However, whether SDB predicts increase in BMI is not well defined. Data from the Sleep Heart Health Study (SHHS) were analyzed to determine whether SDB predicts longitudinal increase in BMI, adjusted for confounding factors.
Methods: A full-montage unattended home polysomnogram (PSG) and body anthropometric measurements were obtained approximately five years apart in 3001 participants. Apnea-hypopnea index (AHI) was categorized using clinical thresholds: < 5 (normal), ≥ 5 to <15 (mild sleep apnea), and ³ 15 (moderate to severe sleep apnea). Linear regression was used to examine the association between the three AHI groups and increased BMI. The model included age, gender, race, baseline BMI, and change in AHI as covariates.
Results: Mean (SD) age was 62.2 years (10.14), 55.2% were female and 76.1% were Caucasian. Five-year increase in BMI was modest with a mean (SD) change of 0.53 (2.62) kg/m2 (p=0.071). A multivariate regression model showed that subjects with a baseline AHI between 5-15 had a mean increase in BMI of 0.22 kg/m2 (p=0.055) and those with baseline AHI ≥ 15 had a BMI increase of 0.51 kg/m2 (p<0.001) compared to those with baseline AHI of <5.
Conclusion: Our findings suggest that there is a positive association between severity of SDB and subsequent increased BMI over approximately 5 years. This observation may help explain why persons with SDB have difficulty losing weight.
Key Words: Sleep Apnea, Weight Gain, Obesity
Abbreviation List: PSG-polysomnogram, SDB-sleep disordered breathing, AHI-apnea hypopnea index, SHHS-Sleep Heart Health Study, BMI-body mass index, SD-standard deviation, SEM-standard error of the mean, ANOVA-analysis of variance
Introduction
There is overwhelming epidemiological and clinical data indicating that obesity is a risk factor for sleep disordered breathing (SDB).1-8 The association between obesity and SDB is substantial, with high body mass index (BMI) contributing to moderate to severe SDB in 58% of affected persons.9 The effect of obesity is greater in men than women1,10-12 although it decreases with increasing age.6,7 In addition, weight loss has been demonstrated to decrease the severity of SDB.10,13,14 Longitudinal data from population studies including the Sleep Heart Health Study (SHHS),10 the Wisconsin Sleep Cohort,15 and the Cleveland Family Study6 have initially focused on the impact of increased weight on SDB severity. However, examination of the opposite causal pathway has yet to be prospectively addressed.
Anecdotally, patients with SDB appear to have more difficulty losing weight than obese patients without SDB. They also report marked weight gain prior to confirmation of their diagnosis. Two small studies support these empiric observations.5,16 Given this limited information on the impact of SDB on BMI, data from SHHS was analyzed to examine the impact of SDB on BMI after controlling for change in AHI and severity of SDB.
Methods
Study Design and Population. The SHHS is a multi-center, community-based prospective cohort study of the natural history and cardiovascular consequences of SDB. Details of the study design, sampling, and procedures have been reported.17 Briefly, between November 1995 and January 1998 participants were recruited from several ongoing prospective cohort studies--the Framingham Offspring and Omni Studies, the Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, the Strong Heart Study, and the cohort studies of respiratory disease in Tucson and of hypertension in New York. Participants were eligible if they were ≥ 40 years of age and were not being treated for sleep apnea with positive pressure therapy, an oral appliance, oxygen, or a tracheostomy. Habitual snorers < 65 years were over sampled to increase the prevalence of obstructive sleep apnea. Subjects were required to provide written consent and the protocol was approved by the institutional review boards of each of the eight investigative sites.
Data Collection. A total of 6,441 subjects completed the baseline polysomnogram (PSG), and 4,586 consented to have a second evaluation approximately five years later. This analysis focuses on the 3,040 participants who had PSG and BMI data at both time points. Data from all 215 participants who had a follow-up PSG from the New York center were excluded because they did not meet quality standards for the follow-up examination. The remaining participants died, were too ill to participate, refused to participate, were lost to follow-up or had incomplete covariate data such as weight. This latter group had a higher percentage of Whites (85%) compared with the study group (75.5%) (p-value <0.001). There also were statistically significant differences in baseline BMI, baseline AHI, and age between the study group compared with the excluded group, however, these differences were very small and were not clinically significant. There was no gender difference between the two groups.
Weight was measured on the night of the PSG examination with the participant in light clothes on a calibrated portable scale. Height was obtained at the baseline home visit if not already measured within + 3 months of the parent study. BMI was calculated as weight in kilograms divided by the square of height in meters. Baseline height was used for baseline and follow-up BMI calculations. Age, sex, and ethnicity were self-reported.
The PSG was conducted using a portable monitor (PS-2 System; Compumedics Limited, Abbotsford, Victoria, Australia), using methods previously described.18 Apnea was present if there was an absence or near absence of airflow or thoracoabdominal movement (at least < 25% of baseline) for > 10 seconds. Hypopnea was defined as a decrease in the amplitude of the airflow or thoracoabdominal movement below 70% of baseline for > 10 seconds. The apnea-hypopnea index (AHI) was calculated as the number of apnea and hypopnea events, each associated with at least a 4% decrease in oxygen saturation, divided by total sleep time in hours.
Results
Participant characteristics are provided in Table 1.
Table 1: Characteristics of participants of the Sleep Heart Health Study cohort with complete baseline and follow-up polysomnography and weight measurements as a function of sleep apnea severity.
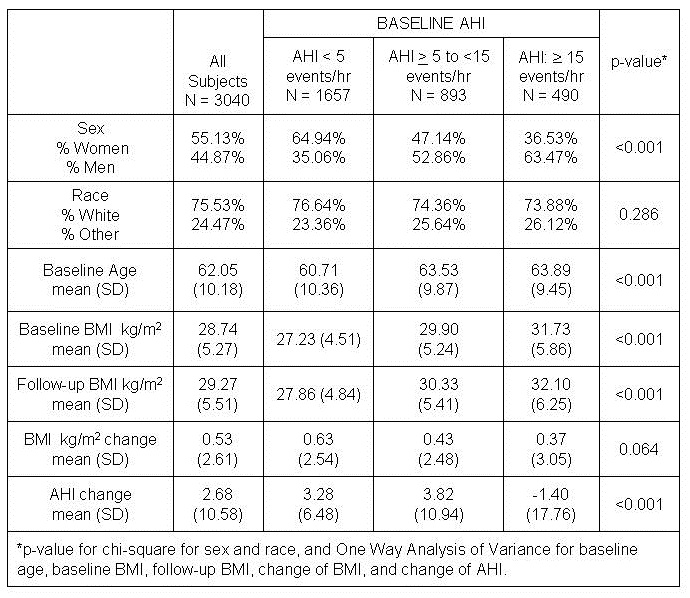
As expected, women were over-represented in the baseline AHI < 5 group (64.9%) and men were over-represented in the AHI ³ 15 group (63.5%) (p<0.001). Baseline BMI increased as baseline AHI severity increased. Overall unadjusted five-year increase in BMI was modest with a mean (SD) BMI change of 0.53 (2.61) kg/m2. The unadjusted five-year increase in BMI was 0.63 (2.54) kg/m2 for those with baseline AHI < 5, 0.43 (2.48) kg/m2 among those with AHI ≥ 5 to < 15 and 0.37 (3.05) kg/m2 for the AHI group ≥ 15. These values were not statistically different from each other.
A multivariate regression model was constructed predicting five-year change in BMI by baseline AHI category adjusted for age, gender, race, baseline BMI, and AHI change (Table 2).
Table 2: Adjusted β coefficients of BMI change according to AHI and continuous variables in the Sleep Heart Health Study*.
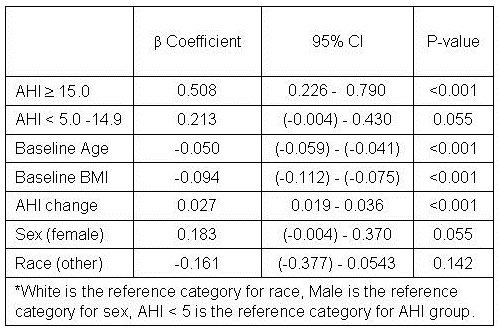
Compared to baseline AHI group of < 5, those with AHI between ≥ 5 to < 15 had a mean adjusted increase in BMI of 0.21 that approached statistical significance (p=0.055). However, those with AHI ≥ 15 had a statistically significant adjusted BMI increase of 0.51 (p<0.001). Younger age, lower baseline BMI and greater AHI change also were associated with a larger BMI increase. There was a trend for women to have a greater increase in BMI, but no effect of race was observed. However, the model only accounted for 7% of the total variance. Adjusted means by baseline AHI group are displayed graphically in Figure 1.
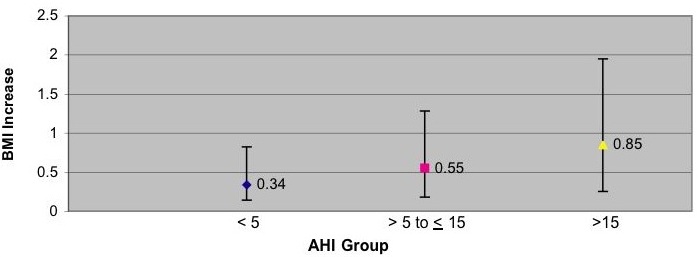
Figure 1: Estimated Adjusted Means of BMI increase according to AHI in the Sleep Heart Health Study. Data are adjusted for baseline age (continuous), race (categorical), gender (categorical), baseline BMI (continuous), change in AHI (continuous). Covariates fixed at: baseline BMI = 28.7, baseline age 62.1, change in RDI = 2.7. Bars represent 95% confidence intervals.
Discussion
Our findings indicate that there is a positive association between severity of SDB and five-year increase in BMI. The finding was demonstrated after controlling for key covariates including age, gender, race, baseline BMI, and AHI change. This observation may help explain the difficulty patients with SDB have in trying to lose weight.
Two previous small studies have demonstrated a positive association between newly diagnosed SDB and weight gain. A retrospective study by Phillips et al. compared one-year weight histories of 53 men and women patients who were recently diagnosed with SDB with 24 control subjects matched for gender, age, BMI and percent body fat.5 Subjects in that study were somewhat younger than the SHHS cohort with an age difference of approximately 10 years. The SDB among subjects in the previous study tended to be moderate to severe with mean ± SEM AHI 33 ± 5 /h for men and 37 ± 10 /h for women. Mean ± SEM of BMI at time of diagnosis was somewhat higher than in the SHHS with 35 ± 1 kg/m2 for men and 44 ± 2 kg/m2 for women. Men and women patients with SDB had reported a recent weight gain of 7.4 ± 1.5 kg compared with a weight loss of 0.5 ± 1.7 kg (p=0.001) in obese controls without SDB. However, given the design of this study it is not possible to determine whether weight gain contributed to the onset of SDB or was a result of SDB. The study was also limited by reliance on self-report of weight gain history.
Another study by Traviss et al. prospectively evaluated 49 obese patients with newly diagnosed SDB.16 Mean ± SD of AHI at diagnosis was severe at 45 ± 27 /h. BMI at diagnosis was elevated at 36.5 ± 6.2 kg/m2. Of the 49 subjects, 43 could estimate the duration of their symptoms with 84% reporting weight gain since becoming symptomatic. Weight gain was relatively large, with a reported 17 ± 15 kg over 5.3 ± 4.8 years. However, this study was limited by the lack of a control group and reliance on self-report of weight history. These two small studies, in addition to our findings, suggest that there is an association between SDB and increased BMI.
Interestingly, unadjusted BMI change in our study was quite modest and not statistically different as a function of SDB severity. However, BMI change over time is a complex phenomenon influenced by several variables. A large (29,799 subjects) prospective study examining 5-year change in weight in a multi-ethnic cohort of men and women explored several of these relationships.19 In that study, younger men and to a greater degree, younger women were at greater risk for weight gain compared to older adults. This is consistent with our initial findings. In addition, there was a trend for women in the higher baseline BMI categories of ‘overweight’ (BMI >25– 30 kg/m2) and ‘obese’ (BMI >30 kg/m2) in the aforementioned cohort to gain more weight than men in the higher baseline BMI categories. In order to more precisely examine the effect of AHI on weight change, we controlled for these confounders in our final multivariate model thus resulting in the finding of an increase in BMI as a function of SDB severity in this study.
Several mechanisms could explain why SDB contributes to increased BMI. First, persons with SDB may have a reduction in the quantity and quality of their sleep. Recent data indicate that insufficient sleep may be a risk factor for obesity.20 Experimental sleep restriction increases ghrelin and reduces leptin production favoring appetite enhancement,21 a finding that also has been observed in a large population cohort.22 Second, those with SDB may eat a diet that favors weight gain. In support of this hypothesis, sleep restriction has been shown to increase craving for calorie dense food with high carbohydrate content. The Apnea Positive Pressure Long-Term Efficacy Study (APPLES) demonstrated that those with severe SDB consumed a diet higher in cholesterol, protein, total fat and total saturated fatty acids, even after adjusting for BMI, age, and daytime sleepiness.23 Third, a cardinal symptom of SDB is excessive daytime sleepiness. Thus, it is possible that persons with SDB engage in less physical activity because they are too fatigued to exercise. Data from APPLES indicate that recreational physical activity is less in those with SDB. However, this finding appears to be principally explained by concomitant obesity.
Weight loss frequently results in an improvement and sometimes resolution in SDB. This is most evident in those who undergo bariatric surgical procedures.24,25 Persons with SDB are frequently counseled to treat their SDB by losing weight through diet and exercise,26 an approach that is usually unsuccessful.25 Failure to primarily address SDB in conjunction with a weight reduction program may diminish the latter’s success. However, evidence to date indicates that treatment of SDB does not consistently result in weight loss. In a sample of clinical patients with SDB, treatment with CPAP did not result in weight loss. Moreover, in female patients, there was actually an increase in weight.27 In addition, consistent weight reduction was not observed in a small number of patients with severe OSA who underwent tracheostomy.28 Thus, it appears that weight gain engendered by the presence of OSA is not easily reversed despite therapy. Prospective studies will be required to determine whether primary treatment for OSA enhances weight loss programs in those with OSA.
Although this analysis demonstrated a positive association of severity of SDB on five-year increase in BMI, there are several caveats that deserve consideration. The BMI of participants tended to be lower than that seen in clinical SDB populations and a relatively small number of subjects had large changes in BMI. As previously noted, the mean BMI increase was, at best, quite modest. When converted for illustrative purposes to weight using an average height of 167 cm of the participants, those with an AHI between ≥ 5 to < 15 had a mean adjusted increase in BMI of 0.21 kg/m2 equal to 0.59 kg or 1.30 lbs. Similarly, those with AHI ≥ 15 had an adjusted BMI increase of 0.51 kg/m2 equal to 1.42 kg or 3.13 lbs. Thus, the magnitude of the changes we observed may not be applicable to clinical populations where patients with SDB may have a higher BMI. In addition, it is not known when the participants developed SDB, thus definitive inference of causality cannot be made. However, following a large undiagnosed cohort over an extended period of time to determine incidence of SDB onset and subsequent change in weight would be exceedingly difficult and costly. Additionally, the model only accounted for a small amount of the total variance in five-year BMI increase, suggesting that there are likely other unmeasured variables influencing the amount of BMI increase over time in this cohort. Finally, while not statistically significant, the unadjusted mean change in BMI was slightly less in the high RDI group in comparison to the lower RDI groups. This observation underscores the biological complexity of the interactions among weight change, SDB, age, gender and other factors.
In conclusion, our findings suggest that although weight gain is a risk factor for developing or worsening SDB, SDB may, in a reciprocal fashion, lead to increased weight gain. This may help explain why patients with SDB find it difficult to lose weight.
Acknowledgements
This work was supported by National Heart, Lung and Blood Institute cooperative agreements U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53934 (University of Minnesota), U01HL53931 (New York University), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL63463 (Case Western Reserve University), and U01HL63429 (Missouri Breaks Research).
Sleep Heart Health Study (SHHS) acknowledges the Atherosclerosis Risk in Communities Study (ARIC), the Cardiovascular Health Study (CHS), the Framingham Heart Study (FHS), the Cornell/Mt. Sinai Worksite and Hypertension Studies, the Strong Heart Study (SHS), the Tucson Epidemiologic Study of Airways Obstructive Diseases (TES) and the Tucson Health and Environment Study (H&E) for allowing their cohort members to be part of the SHHS and for permitting data acquired by them to be used in the study. SHHS is particularly grateful to the members of these cohorts who agreed to participate in SHHS as well. SHHS further recognizes all of the investigators and staff who have contributed to its success. A list of SHHS investigators, staff and their participating institutions is available on the SHHS website, www.jhucct.com/shhs.
The opinions expressed in the paper are those of the author(s) and do not necessarily reflect the views of the Indian Health Service.
These data have been presented in part at the Annual Meeting of the Associated Professional Sleep Societies, June 11, 2009, Seattle, WA.
References
- Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: Effects of gender. Am J Respir Crit Care Med 2001;163:608-13.
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. prevalence and severity. Am J Respir Crit Care Med 1998;157:144-8.
- Carmelli D, Swan GE, Bliwise DL. Relationship of 30-year changes in obesity to sleep-disordered breathing in the western collaborative group study. Obes Res 2000;8:632-7.
- Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001;163:685-9.
- Phillips BG, Hisel TM, Kato M, Pesek CA, Dyken ME, Narkiewicz K, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens 1999;17:1297-300.
- Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: The relative importance of risk factors in the development of sleep-disordered breathing. JAMA 2003;289:2230-7.
- Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: The sleep heart health study. Arch Intern Med 2002;162:893-900.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5.
- Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol 2005;99:1592-9.
- Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: The sleep heart health study. Arch Intern Med 2005;165:2408-13.
- Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged chinese women in hong kong: Prevalence and gender differences. Chest 2004;125:127-34.
- Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD. Body fat distribution and sleep apnea severity in women. Chest 1995;107:362-6.
- Barvaux VA, Aubert G, Rodenstein DO. Weight loss as a treatment for obstructive sleep apnoea. Sleep Med Rev 2000;4:435-52.
- Grunstein RR, Stenlof K, Hedner JA, Peltonen M, Karason K, Sjostrom L. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep 2007;30:703-10.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-21.
- Traviss KA, Barr SI, Fleming JA, Ryan CF. Lifestyle-related weight gain in obese men with newly diagnosed obstructive sleep apnea. J Am Diet Assoc 2002;102:703-6.
- Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, et al. The sleep heart health study: Design, rationale, and methods. Sleep 1997;20:1077-85.
- Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. sleep heart health research group. Sleep1998;21:759-67.
- Ball K, Crawford D, Ireland P, Hodge A. Patterns and demographic predictors of 5-year weight change in a multi-ethnic cohort of men and women in australia. Public Health Nutr 2003;6:269-81.
- Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: A 6-year prospective study from the Quebec Family Study. Sleep 2008;31:517-23.
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846-50.
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62.
- Vasquez MM, Goodwin JL, Drescher AA, Smith TW, Quan SF. Associations of dietary intake and physical activity with sleep disordered breathing in the apnea positive pressure long-term efficacy study (APPLES). J Clin Sleep Med 2008;4:411-8.
- Rasheid S, Banasiak M, Gallagher SF, Lipska A, Kaba S, Ventimiglia D, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
- Fritscher LG, Canani S, Mottin CC, Fritscher CC, Berleze D, Chapman K, et al. Bariatric surgery in the treatment of obstructive sleep apnea in morbidly obese patients. Respiration 2007;74:647-52.
- Veasey SC, Guilleminault C, Strohl KP, Sanders MH, Ballard RD, Magalang UJ. Medical therapy for obstructive sleep apnea: A review by the medical therapy for obstructive sleep apnea task force of the standards of practice committee of the american academy of sleep medicine. Sleep 2006;29:1036-44.
- Redenius R, Murphy C, O'Neill E, Al-Hamwi M, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med 2008;4:205-9.
- Haapaniemi JJ, Laurikainen EA, Halme P, Antila J. Long-term results of tracheostomy for severe obstructive sleep apnea syndrome. ORL J Otorhinolaryngol Relat Spec 2001;63:131-6.

Reader Comments (1)
Erratum: In the original PDF version of this manuscript, table 2 was included twice, once under table 1. This was corrected on 1-5-12.